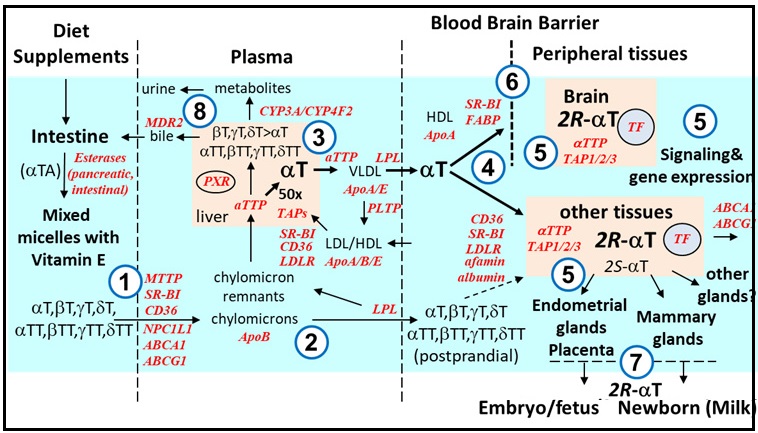
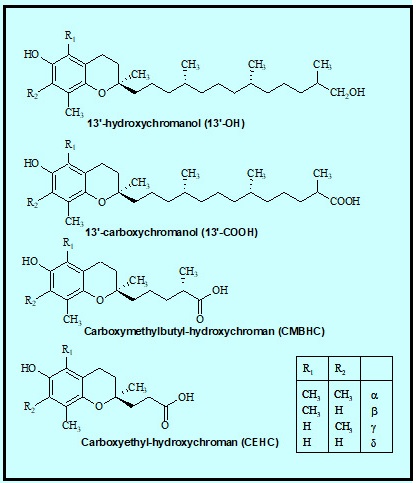
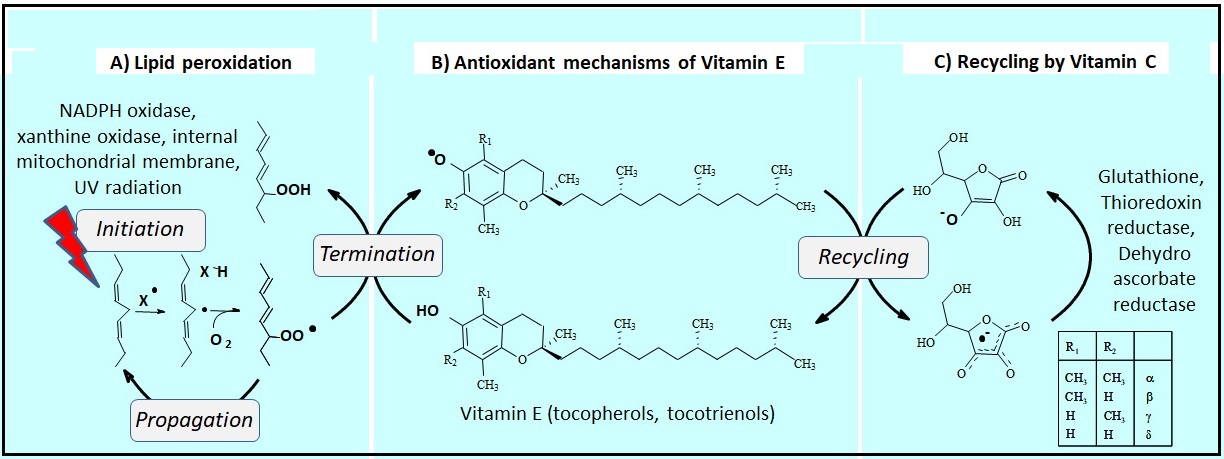
Abdo, K.M., Rao, G., Montgomery, C.A., Dinowitz, M., Kanagalingam, K., 1986. Thirteen-week toxicity study of d-alpha-tocopheryl acetate (vitamin E) in Fischer 344 rats. Food Chem Toxicol 24 (10-11), 1043-1050.
Abner, E.L., Schmitt, F.A., Mendiondo, M.S., Marcum, J.L., Kryscio, R.J., 2011. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 4 (2), 158-170.
Alcala, M., Bolado, V.E., Sanchez-Vera, I., Clapes, S., Dasi, F., Saez, G., Carrera, E., Alvarez-Gallego, F., Loeken, M.R., Viana, M., 2021. Prevention of Teratogenesis in Pregnancies of Obese Rats by Vitamin E Supplementation. Antioxidants (Basel) 10 (8).
Alcala, M., Calderon-Dominguez, M., Bustos, E., Ramos, P., Casals, N., Serra, D., Viana, M., Herrero, L., 2017. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep 7 (1), 16082.
Arai, H., Kono, N., 2021. alpha-tocopherol transfer protein (alpha-TTP). Free Radic Biol Med 176, 162-175.
Asbaghi, O., Sadeghian, M., Nazarian, B., Sarreshtedari, M., Mozaffari-Khosravi, H., Maleki, V., Alizadeh, M., Shokri, A., Sadeghi, O., 2020. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomized clinical trials. Sci Rep 10 (1), 17234.
Azzi, A., 2018. Many tocopherols, one vitamin E. Mol Aspects Med 61, 92-103.
Azzi, A., 2019. tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol 26, 101259.
Azzi, A., Atkinson, J., Ozer, N.K., Manor, D., Wallert, M., Galli, F., 2023. Vitamin E discussion forum position paper on the revision of the nomenclature of vitamin E. Free Radic Biol Med 207, 178-180.
Azzi, A., Gysin, R., Kempna, P., Ricciarelli, R., Villacorta, L., Visarius, T., Zingg, J.M., 2003. The role of alpha-tocopherol in preventing disease: from epidemiology to molecular events. Mol Aspects Med 24, 325-336.
Azzi, A., Zingg, J.M., 2006. The role and importance of vitamin E for general health. Clinical Dermatology; Retinoids and other treatments 22, 26-29.
Bartolini, D., Marinelli, R., Giusepponi, D., Galarini, R., Barola, C., Stabile, A.M., Sebastiani, B., Paoletti, F., Betti, M., Rende, M., Galli, F., 2021. Alpha-tocopherol Metabolites (the Vitamin E Metabolome) and Their Interindividual Variability during Supplementation. Antioxidants (Basel) 10 (2).
Bartolini, D., Marinelli, R., Stabile, A.M., Frammartino, T., Guerrini, A., Garetto, S., Lucci, J., Migni, A., Zatini, L., Marcantonini, G., Rende, M., Galli, F., 2022. Wheat germ oil vitamin E cytoprotective effect and its nutrigenomics signature in human hepatocyte lipotoxicity. Heliyon 8 (9), e10748.
Baschong, W., Artmann, C., Hueglin, D., Roeding, J., 2001. Direct evidence for bioconversion of vitamin E acetate into vitamin E: an ex vivo study in viable human skin. J Cosmet Sci 52 (3), 155-161.
Bauer, S.R., Richman, E.L., Sosa, E., Weinberg, V., Song, X., Witte, J.S., Carroll, P.R., Chan, J.M., 2013. Antioxidant and vitamin E transport genes and risk of high-grade prostate cancer and prostate cancer recurrence. Prostate 73 (16), 1786-1795.
Behrens, W.A., Thompson, J.N., Madere, R., 1982. Distribution of alpha-tocopherol in human plasma lipoproteins. Am. J. Clin. Nutr. 35, 691-696.
Belcher, J.D., Balla, J., Balla, G., Jacobs, D.R., Jr., Gross, M., Jacob, H.S., Vercellotti, G.M., 1993. Vitamin E, LDL, and endothelium. Brief oral vitamin supplementation prevents oxidized LDL-mediated vascular injury in vitro. Arterioscler Thromb 13, 1779-1789.
Bellizzi, M.C., Franklin, M.F., Duthie, G.G., James, W.P.T., 1994. Vitamin E and coronary heart disease: the European paradox. European Journal of Clinical Nutrition 48, 822-831.
Betancor-Fernandez, A., Sies, H., Stahl, W., Polidori, M.C., 2002. In vitro antioxidant activity of 2,5,7,8-tetramethyl-2-(2'-carboxyethyl)-6-hydroxychroman (alpha-CEHC), a vitamin E metabolite. Free Radic Res 36, 915-921.
Bieri, J.G., Tolliver, T.J., Catignani, G.L., 1979. Simulta- neous determination of α-tocopherol and retinol in plasma or red cells by high pressure liquid chro- matography. American Journal of Clinical Nutrition 32, 2143-2149.
Binder, H.J., Spiro, H.M., 1967. tocopherol deficiency in man. Am J Clin Nutr 20, 594-603.
Birringer, M., Drogan, D., Brigelius-Flohe, R., 2001. tocopherols are metabolized in HepG2 cells by side chain omega-oxidation and consecutive beta-oxidation. Free Radic. Biol. Med. 31, 226-232.
Birringer, M., Lorkowski, S., 2019. Vitamin E: Regulatory role of metabolites. IUBMB Life 71 (4), 479-486.
Bjelakovic, G., Nikolova, D., Gluud, L.L., Simonetti, R.G., Gluud, C., 2007. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama 297, 842-857.
Blount, B.C., Karwowski, M.P., Shields, P.G., Morel-Espinosa, M., Valentin-Blasini, L., Gardner, M., Braselton, M., Brosius, C.R., Caron, K.T., Chambers, D., Corstvet, J., Cowan, E., De Jesus, V.R., Espinosa, P., Fernandez, C., Holder, C., Kuklenyik, Z., Kusovschi, J.D., Newman, C., Reis, G.B., Rees, J., Reese, C., Silva, L., Seyler, T., Song, M.A., Sosnoff, C., Spitzer, C.R., Tevis, D., Wang, L., Watson, C., Wewers, M.D., Xia, B., Heitkemper, D.T., Ghinai, I., Layden, J., Briss, P., King, B.A., Delaney, L.J., Jones, C.M., Baldwin, G.T., Patel, A., Meaney-Delman, D., Rose, D., Krishnasamy, V., Barr, J.R., Thomas, J., Pirkle, J.L., Lung Injury Response Laboratory Working, G., 2019. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med.
Blum, S., Vardi, M., Brown, J.B., Russell, A., Milman, U., Shapira, C., Levy, N.S., Miller-Lotan, R., Asleh, R., Levy, A.P., 2010. Vitamin E reduces cardiovascular disease in individuals with diabetes mellitus and the haptoglobin 2-2 genotype. Pharmacogenomics 11 (5), 675-684.
Bolton-Smith, C., Casey, C.E., Gey, K.F., Smith, W.C., Tunstall-Pedoe, H., 1991. Antioxidant vitamin intakes assessed using a food-frequency questionnaire: correlation with biochemical status in smokers and non-smokers. Br J Nutr 65 (3), 337-346.
Boonnoy, P., Karttunen, M., Wong-Ekkabut, J., 2017. Alpha-tocopherol inhibits pore formation in oxidized bilayers. Phys Chem Chem Phys 19 (8), 5699-5704.
Boonnoy, P., Karttunen, M., Wong-Ekkabut, J., 2018. Does alpha-tocopherol Flip-Flop Help to Protect Membranes Against Oxidation? J Phys Chem B 122 (45), 10362-10370.
Borel, P., Desmarchelier, C., 2016. Genetic Variations Involved in Vitamin E Status. Int J Mol Sci 17 (12).
Borel, P., Desmarchelier, C., Nowicki, M., Bott, R., Tourniaire, F., 2015. Can genetic variability in alpha-tocopherol bioavailability explain the heterogeneous response to alpha-tocopherol supplements? Antioxid Redox Signal.
Borel, P., Lietz, G., Goncalves, A., Szabo de Edelenyi, F., Lecompte, S., Curtis, P., Goumidi, L., Caslake, M.J., Miles, E.A., Packard, C., Calder, P.C., Mathers, J.C., Minihane, A.M., Tourniaire, F., Kesse-Guyot, E., Galan, P., Hercberg, S., Breidenassel, C., Gonzalez Gross, M., Moussa, M., Meirhaeghe, A., Reboul, E., 2013a. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr 143 (4), 448-456.
Borel, P., Preveraud, D., Desmarchelier, C., 2013b. Bioavailability of vitamin E in humans: an update. Nutr Rev 71 (6), 319-331.
Bortolotti, A., Lucchini, G., Barzago, M.M., Stellari, F., Bonati, M., 1993. Simultaneous determination of retinol, α-tocopherol and retinyl palmitate in plasma of premature newborns by reversed-phase high-per- formance liquid chromatography. Journal of Chro- matography 617, 313-317.
Brigelius-Flohe, R., 2003. Vitamin E and drug metabolism. Biochem. Biophys. Res. Commun. 305, 737-740.
Brigelius-Flohe, R., Kelly, F.J., Salonen, J.T., Neuzil, J., Zingg, J.M., Azzi, A., 2002. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr 76 (4), 703-716.
Brigelius-Flohe, R., Traber, M.G., 1999. Vitamin E: function and metabolism. FASEB. J. 13, 1145-1155.
Burton, G.W., Traber, M.G., 1990. Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu. Rev. Nutr. 10, 357-382.
Burton, G.W., Traber, M.G., Acuff, R.V., Walters, D.N., Kayden, H., Hughes, L., Ingold, K.U., 1998. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am. J. Clin. Nutr. 67, 669-684.
Cavalier, L., Ouahchi, K., Kayden, H.J., Di Donato, S., Reutenauer, L., Mandel, J.L., Koenig, M., 1998. Ataxia with isolated vitamin E deficiency: heterogeneity of mutations and phenotypic variability in a large number of families. Am. J. Hum. Genet. 62, 301-310.
Chen, B., McClements, D.J., Decker, E.A., 2011. Minor components in food oils: a critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit Rev Food Sci Nutr 51 (10), 901-916.
Chiku, S., Hamamura, K., Nakamura, T., 1984. Novel urinary metabolite of d-delta-tocopherol in rats. J. Lipid. Res. 25, 40-48.
Clemente, H.A., Ramalho, H.M., Lima, M.S., Grilo, E.C., Dimenstein, R., 2015. Maternal supplementation with natural or synthetic vitamin E and its levels in human colostrum. J Pediatr Gastroenterol Nutr 60 (4), 533-537.
Constantinou, C., Papas, A., Constantinou, A.I., 2008. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int J Cancer 123 (4), 739-752.
Coulter, I.D., Hardy, M.L., Morton, S.C., Hilton, L.G., Tu, W., Valentine, D., Shekelle, P.G., 2006. Antioxidants vitamin C and vitamin e for the prevention and treatment of cancer. J Gen Intern Med 21, 735-744.
Cynamon, H.A., Isenberg, J.N., 1987. Characterization of vitamin E status in cholestatic children by con- ventional laboratory standards and a new functional assay. Journal of Pediatric Gastroenterology and Nutrition 6, 46-50.
Cynamon, H.A., Isenberg, J.N., Nguyen, C., 1985. Eryth- rocyte malondialdehyde release in vitro: a func- tional measure of vitamin E status. Clinica Chimica Acta 151, 169-176.
Devaraj, S., Hugou, I., Jialal, I., 2001. Alpha-tocopherol decreases CD36 expression in human monocyte-derived macrophages. J. Lipid. Res. 42, 521-527.
Dong, L., Gopalan, V., Holland, O., Neuzil, J., 2020. Mitocans Revisited: Mitochondrial Targeting as Efficient Anti-Cancer Therapy. Int J Mol Sci 21 (21).
Driskell, W.J., Neese, J.W., Bryant, C.C., Bashor, M., 1982. Measurement of vitamin A and vitamin E in human serum by high performance liquid chromatography. Journal of Chromatography 231, 439-444.
Duthie, G.G., Arthur, J.J.R., W.P, 1991. Effects of smoking and vitamin E on blood antioxidant sta- tus. American Journal of Clinical Nutrition 53, 1061-1063.
Efsa Panel on Nutrition, N.F., Food, A., Turck, D., Bohn, T., Castenmiller, J., de Henauw, S., Hirsch-Ernst, K.I., Knutsen, H.K., Maciuk, A., Mangelsdorf, I., McArdle, H.J., Pentieva, K., Siani, A., Thies, F., Tsabouri, S., Vinceti, M., Traber, M.G., Vrolijk, M., Bercovici, C.M., de Sesmaisons Lecarre, A., Fabiani, L., Karavasiloglou, N., Mendes, V., Valtuena Martinez, S., Naska, A., 2024. Scientific opinion on the tolerable upper intake level for vitamin E. EFSA J 22 (8), e8953.
Eggersdorfer, M., Schmidt, K., Peter, S., Richards, J., Winklhofer-Roob, B., Hahn, A., Obermuller-Jevic, U., 2024. Vitamin E: Not only a single stereoisomer. Free Radic Biol Med 215, 106-111.
El-Sohemy, A., Baylin, A., Kabagambe, E., Ascherio, A., Spiegelman, D., Campos, H., 2002. Individual car- otenoid concentrations in adipose tissue and plasma as biomarkers of dietary intake. American Journal of Clinical Nutrition 76, 172-179.
Epler, K.S., Ziegler, R.G., Craft, N.E., 1993. Liquid chromatographic method for the determination of carotenoids, retinoids and tocopherols in human serum and in food. Journal of Chromatography 619, 37-48.
Erhardt, J.G., Mack, H., Sobeck, U., Biesalski, H.K., 2002. β-carotene and α-tocopherol concentration and anti- oxidant status in buccal mucosal cells and plasma after oral supplementation. British Journal of Nutri- tion 87, 471-475.
Evans, H.M., Bishop, K.S., 1922. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 56, 650-651.
Fanali, G., Fasano, M., Ascenzi, P., Zingg, J.-M., Azzi, A., 2013. α-tocopherol binding to human serum albumin.
Farrell, P.M., Bieri, J.G., 1975. Megavitamin E supple- mentation in man. American Journal of Clinical Nutrition 28, 1381-1386.
Farrell, P.M., Bieri, J.G., Fraantoni, J.F., Wood, R.E., Sant’Agnese, P.A., 1977. The occurrence and ef- fects of human vitamin E deficiency: a study in patients with cystic fibrosis. Journal of Clinical In vestigation 6, 233-241.
Farrell, P.M., Levine, S.L., Murphy, M.D., Adams, A., 1978. Plasma tocopherol levels and tocopherol-lipid re- lationships in a normal population of children as compared to healthy adults. American Journal of Clinical Nutrition 31, 1720-1726.
Finch, S., Doyle, W., Lowe, C., Bates, C.J., Prentice, A., Smithers, G., Clarke, P.C., 1998. National Diet and Nutrition Survey: People Aged 65 Years or Over, Report of the Diet and Nutrition Survey. The Stationery Office, London.
Food and Nutrition Board, I.o.M., 2000. Vitamin E, in: PRESS, N.A. (Ed.), Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (2000). Institute of Medicine (IOM), Washington, D.C., pp. 186-283.
Ford, E.S., Sowell, A., 1999. Serum α-tocopherol sta- tus in the United States population: findings from the Third National Health and Nutrition Examina- tion Survey. American Journal of Epidemiology 1, 290-300.
Galli, F., Azzi, A., Birringer, M., Cook-Mills, J.M., Eggersdorfer, M., Frank, J., Cruciani, G., Lorkowski, S., Ozer, N.K., 2016. Vitamin E: Emerging aspects and new directions. Free Radic Biol Med 102, 16-36.
Gascon-Vila, P., Garcia-Closas, R., Serra-Majem, L., Pastor, M.C., Ribas, L., Ramon, J.M., Marine-Font, A., Salleras, L., 1997. Determinants of the nutritional status of vitamin E in a non-smoking Mediterranean population. Analysis of the effect of vitamin E intake, alcohol consumption and body mass index on the serum alpha-tocopherol concentration. Eur J Clin Nutr 51 (11), 723-728.
Gaur, S., Kuchan, M.J., Lai, C.S., Jensen, S.K., Sherry, C.L., 2017. supplementation with RRR- or all-rac-alpha-tocopherol differentially Affects the alpha-tocopherol Stereoisomer Profile in the Milk and Plasma of Lactating Women. J Nutr 147 (7), 1301-1307.
Gavin, P.D., El-Tamimy, M., Keah, H.H., Boyd, B.J., 2016. tocopheryl phosphate mixture (TPM) as a novel lipid-based transdermal drug delivery carrier: formulation and evaluation. Drug Deliv Transl Res.
Gerss, J., Kopcke, W., 2009. The questionable association of vitamin E supplementation and mortality--inconsistent results of different meta-analytic approaches. Cell Mol Biol (Noisy-le-grand) 55 Suppl, OL1111-1120.
Ghone, R.A., Suryakar, A.N., Kulhalli, P.M., Bhagat, S.S., Padalkar, R.K., Karnik, A.C., Hundekar, P.S., Sangle, D.A., 2013. A study of oxidative stress biomarkers and effect of oral antioxidant supplementation in severe acute malnutrition. J Clin Diagn Res 7 (10), 2146-2148.
Giusepponi, D., Galarini, R., Barola, C., Torquato, P., Bartolini, D., Moretti, S., Saluti, G., Gioiello, A., Libetta, C., Galli, F., 2019. LC-MS/MS assay for the simultaneous determination of tocopherols, polyunsaturated fatty acids and their metabolites in human plasma and serum. Free Radic Biol Med 144, 134-143.
Giusepponi, D., Torquato, P., Bartolini, D., Piroddi, M., Birringer, M., Lorkowski, S., Libetta, C., Cruciani, G., Moretti, S., Saluti, G., Galli, F., Galarini, R., 2017. Determination of tocopherols and their metabolites by liquid-chromatography coupled with tandem mass spectrometry in human plasma and serum. Talanta 170, 552-561.
Glatz, J.F., Angin, Y., Steinbusch, L.K., Schwenk, R.W., Luiken, J.J., 2012. CD36 as a target to prevent cardiac lipotoxicity and insulin resistance. Prostaglandins Leukot Essent Fatty Acids.
Gohil, K., Godzdanker, R., O'Roark, E., Schock, B.C., Kaini, R.R., Packer, L., Cross, C.E., Traber, M.G., 2004. Alpha-tocopherol transfer protein deficiency in mice causes multi-organ deregulation of gene networks and behavioral deficits with age. Ann N Y Acad Sci 1031, 109-126.
Gohil, K., Schock, B.C., Chakraborty, A.A., Terasawa, Y., Raber, J., Farese, R.V., Jr., Packer, L., Cross, C.E., Traber, M.G., 2003. Gene expression profile of oxidant stress and neurodegeneration in transgenic mice deficient in alpha-tocopherol transfer protein. Free Radic Biol Med 35, 1343-1354.
Gotoda, T., Arita, M., Arai, H., Inoue, K., Yokota, T., Fukuo, Y., Yazaki, Y., Yamada, N., 1995. Adult-onset spino- cerebellar dysfunction caused by a mutation in the gene for alpha-tocopherol-transfer protein. New England Journal of Medicine 333, 1313-1318.
Grebenstein, N., Schumacher, M., Graeve, L., Frank, J., 2014. alpha-tocopherol transfer protein is not required for the discrimination against gamma-tocopherol in vivo but protects it from side-chain degradation in vitro. Mol Nutr Food Res 58 (5), 1052-1060.
Gregory, J., Foster, K., Tyler, H., Wiseman, M., 1990. The Dietary and Nutritional Survey of British Adults. Her Majesty’s Stationery Office, London.
Gregory, J., Lowe, S., Bates, C.J., Prentice, A., Jackson, L.V., Smithers, G., Wenlock, R., Farron, M., 2000. National Diet and Nutrition Survey: Young People Aged 4 to 18 Years, Report of the Diet and Nutrition Survey. The Stationery Office, London.
Grilo, E.C., Costa, P.N., Gurgel, C.S.S., Beserra, A.F., Almeida, F.N., Dimenstein, R., 2014. Alpha-tocopherol and gamma-tocopherol concentration in vegatable oils. Food Science and Technology 34 (2), 379-385.
Haga, S., Miyaji, M., Nakano, M., Ishizaki, H., Matsuyama, H., Katoh, K., Roh, S.G., 2018. Changes in the expression of alpha-tocopherol-related genes in liver and mammary gland biopsy specimens of peripartum dairy cows. J Dairy Sci 101 (6), 5277-5293.
Halliwell, B., Rafter, J., Jenner, A., 2005. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr 81, 268S-276S.
Han, S.N., Pang, E., Zingg, J.M., Meydani, S.N., Meydani, M., Azzi, A., 2010. Differential effects of natural and synthetic vitamin E on gene transcription in murine T lymphocytes. Arch Biochem Biophys 495 (1), 49-55.
Hassan, H., Hashim, S.A., Itallie, T.B., Sebrell, W.H., 1966. Syndrome in premature infants associated with low plasma vitamin E levels and high polyun- saturated fatty acid diet. American Journal of Clini- cal Nutrition 19, 147-157.
Hathcock, J.N., Azzi, A., Blumberg, J., Bray, T., Dickinson, A., Frei, B., Jialal, I., Johnston, C.S., Kelly, F.J., Kraemer, K., Packer, L., Parthasarathy, S., Sies, H., Traber, M.G., 2005. Vitamins E and C are safe across a broad range of intakes. Am J Clin Nutr 81, 736-745.
Hayashi, D., Mouchlis, V.D., Okamoto, S., Namba, T., Wang, L., Li, S., Ueda, S., Yamanoue, M., Tachibana, H., Arai, H., Ashida, H., Dennis, E.A., Shirai, Y., 2022. Vitamin E functions by association with a novel binding site on the 67 kDa laminin receptor activating diacylglycerol kinase. J Nutr Biochem 110, 109129.
Hempstock, J., Cindrova-Davies, T., Jauniaux, E., Burton, G.J., 2004. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol 2, 58.
Hennig, B., Boissonneault, G.A., Fiscus, L.J., Marra, M.E., 1988. Effect of vitamin E on oxysterol- and fatty acid hydroperoxide-induced changes of repair and permeability properties of cultured endothelial cell monolayers. Int J Vitam Nutr Res 58, 41-47.
Herbeth, B., Chavance, M., Musse, N., Mejean, L., Vernhes, G., 1989. Dietary intake and other determinants of blood vitamins in an elderly population. Euro- pean Journal of Clinical Nutrition 43, 175-186.
Horwitt, M.K., 1960. Vitamin E and lipid metabolism in man. American Journal of Clinical Nutrition 8, 451-461.
Horwitt, M.K., Harvey, C.C., Ch, D., Jr., Searcy, M.T., 1972. Relationship between tocopherol and serum lipid levels for determination of nutritional ade- quacy. Annals of New York Academy of Sciences 203, 223-236.
Hyland, S., Muller, D., Hayton, S., Stoecklin, E., Barella, L., 2006. Cortical gene expression in the vitamin E-deficient rat: possible mechanisms for the electrophysiological abnormalities of visual and neural function. Ann Nutr Metab 50 (5), 433-441.
Imai, E., Tsuji, T., Sano, M., Fukuwatari, T., Shibata, K., 2011. Association between 24 hour urinary alpha-tocopherol catabolite, 2,5,7,8-tetramethyl-2(2'-carboxyethyl)-6-hydroxychroman (alpha-CEHC) and alpha-tocopherol intake in intervention and cross-sectional studies. Asia Pac J Clin Nutr 20 (4), 507-513.
IOS, 2000. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. National Academy Press, Washington, DC, 186-283.
Jack Yang, N.Y., Desai, I.D., 1977. Effect of high levels of dietary vitamin E on hematological indices and biochemical parameters in rats. J Nutr 107 (8), 1410-1417.
Jiang, Q., 2014. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic Biol Med 72, 76-90.
Jiang, Q., 2017. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv Nutr 8 (6), 850-867.
Jiang, Q., 2018. Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms. IUBMB Life.
Jiang, Q., Christen, S., Shigenaga, M.K., Ames, B.N., 2001. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 74, 714-722.
Jishage, K., Arita, M., Igarashi, K., Iwata, T., Watanabe, M., Ogawa, M., Ueda, O., Kamada, N., Inoue, K., Arai, H., Suzuki, H., 2001. Alpha-tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J. Biol. Chem. 276, 1669-1672.
Johnson, L., Schaffer, D., Boggs, T.R., Jr., 1974. The premature infant, vitamin E deficiency and retrolental fibroplasia. Am J Clin Nutr 27 (10), 1158-1173.
Kaempf, D.E., Miki, M., Ogihara, T., Okamoto, R., K, K., Mino, M., 1994. Assessment of vitamin E nutritional status in neonates, infants and children on the basis of α-tocopherol levels in blood com- ponents and buccal mucosal cells. International Journal for Vitamin and Nutrition Research 64, 185-191.
Kalra, V., Grover, J., Ahuja, G.K., Rathi, S., Khurana, D.S., 1998. Vitamin E deficiency and associated neu- rological deficits in children with protein-energy malnutrition. Journal of Tropical Pediatrics 44, 291-295.
Kardinaal, A.F., Veer, P., Brants, H.A., Berg, H., Schoohoven, J., Hermus, R.J., 1995. Relations between antioxidant vitamins in adipose tissue, plas- ma, and diet. American Journal of Epidemiology 141, 440-450.
Kayden, H.J., Hatam, L.J., Traber, M.G., 1983. The meas- urement of nanograms of tocopherol from needle aspiration biopsies of adipose tissue: normal and abetalipoproteinemic subjects. Journal of Lipid Research 24, 652-656.
Kayden, H.J., Traber, M.G., 1993. Absorption, lipoprotein transport, and regulation of plasma concentrations of vitamin E in humans. J. Lipid. Res. 34, 343-358.
Keaney, J.F., Jr., Guo, Y., Cunningham, D., Shwaery, G.T., Xu, A., Vita, J.A., 1996. Vascular incorporation of alpha-tocopherol prevents endothelial dysfunction due to oxidized LDL by inhibiting protein kinase C stimulation. J. Clin. Invest. 98, 386-394.
Kim, H.K., Han, S.N., 2019. Vitamin E: Regulatory role on gene and protein expression and metabolomics profiles. IUBMB Life 71 (4), 442-455.
Kohlschutter, A., Finckh, B., Nickel, M., Bley, A., Hubner, C., 2020. First Recognized Patient with Genetic Vitamin E Deficiency Stable after 36 Years of Controlled Supplement Therapy. Neurodegener Dis 20 (1), 35-38.
Kono, N., Arai, H., 2015. Intracellular transport of fat-soluble vitamins A and E. Traffic 16 (1), 19-34.
Kuchan, M.J., DeMichele, S.J., Schimpf, K.J., Chen, X., 2021. alpha-tocopherol Stereoisomer Profiles in Matched Human Maternal and Umbilical Cord Plasma. Curr Dev Nutr 5 (6), nzab073.
Kuchan, M.J., Jensen, S.K., Johnson, E.J., Lieblein-Boff, J.C., 2016. The naturally occurring alpha-tocopherol stereoisomer RRR-alpha-tocopherol is predominant in the human infant brain. Br J Nutr 116 (1), 126-131.
Kuchan, M.J., Moulton, C.J., Dyer, R.A., Jensen, S.K., Schimpf, K.J., Innis, S.M., 2018. RRR-alpha-tocopherol Is the Predominant Stereoisomer of alpha-tocopherol in Human Milk. Curr Dev Nutr 2 (8), nzy055.
Kuchan, M.J., Ranard, K.M., Dey, P., Jeon, S., Sasaki, G.Y., Schimpf, K.J., Bruno, R.S., Neuringer, M., Erdman, J.W., 2020. Infant Rhesus Macaque Brain alpha-tocopherol Stereoisomer Profile Is differentially Impacted by the Source of alpha-tocopherol in Infant Formula. J Nutr 150 (9), 2305-2313.
Kuzuya, M., Naito, M., Funaki, C., Hayashi, T., Asai, K., Kuzuya, F., 1991. Probucol prevents oxidative injury to endothelial cells. J Lipid Res 32, 197-204.
Landes, N., Pfluger, P., Kluth, D., Birringer, M., Ruhl, R., Bol, G.F., Glatt, H., Brigelius-Flohe, R., 2003. Vitamin E activates gene expression via the pregnane X receptor. Biochem. Pharmacol. 65, 269-273.
Lebold, K.M., Ang, A., Traber, M.G., Arab, L., 2012. Urinary alpha-carboxyethyl hydroxychroman can be used as a predictor of alpha-tocopherol adequacy, as demonstrated in the Energetics Study. Am J Clin Nutr 96 (4), 801-809.
Lecompte, S., Szabo de Edelenyi, F., Goumidi, L., Maiani, G., Moschonis, G., Widhalm, K., Molnar, D., Kafatos, A., Spinneker, A., Breidenassel, C., Dallongeville, J., Meirhaeghe, A., Borel, P., 2011. Polymorphisms in the CD36/FAT gene are associated with plasma vitamin E concentrations in humans. Am J Clin Nutr 93 (3), 644-651.
Lee, P., Ulatowski, L.M., 2019. Vitamin E: Mechanism of transport and regulation in the CNS. IUBMB Life 71 (4), 424-429.
Lehmann, J., 1981. Comparative sensitivities of tocopherol levels of platelets, red blood cells and plasma for estimating vitamin E nutritional status in the rat. Am J Clin Nutr 34 (10), 2104-2110.
Lehmann, J., Rao, D.D., Canary, J.J., Judd, J.T., 1988. Vitamin E and relationships among tocopherols in human plasma, platelets, lymphocytes, and red blood cells. American Journal of Clinical Nutrition 47, 470-474.
Lemcke-Norojarvi, M., Kamal-Eldin, A., Appelqvist, L.A., Dimberg, L.H., Ohrvall, M., Vessby, B., 2001. Corn and sesame oils increase serum gamma-tocopherol concentrations in healthy Swedish women. J. Nutr. 131, 1195-1201.
Lemoyne, M., Gossum, A., Kurian, R., Ostro, M., Axler, J., Jeejeebhoy, K.N., 1987. Breath pentane analysis as an index of lipid peroxidation: a func- tional test of vitamin E status. American Journal of Clinical Nutrition 46, 267-272.
Leng, X., Kinnun, J.J., Marquardt, D., Ghefli, M., Kucerka, N., Katsaras, J., Atkinson, J., Harroun, T.A., Feller, S.E., Wassall, S.R., 2015. alpha-tocopherol Is Well Designed to Protect Polyunsaturated Phospholipids: MD Simulations. Biophys J 109 (8), 1608-1618.
Liqiang, S., Fang-Hui, L., Minghui, Q., Yanan, Y., Haichun, C., 2023. Free fatty acids and peripheral blood mononuclear cells (PBMC) are correlated with chronic inflammation in obesity. Lipids Health Dis 22 (1), 93.
Loncaric, D., Rodriguez, L., Debeissat, C., Touya, N., Labat, V., Villacreces, A., Bouzier-Sore, A.K., Pasquet, J.M., de la Grange, P.B., Vlaski-Lafarge, M., Pavlovic, S., Ivanovic, Z., 2021. alpha-tocopherol Acetate Attenuates Mitochondrial Oxygen Consumption and Maintains Primitive Cells within Mesenchymal Stromal Cell Population. Stem Cell Rev Rep.
Lonn, E., Bosch, J., Yusuf, S., Sheridan, P., Pogue, J., Arnold, J.M., Ross, C., Arnold, A., Sleight, P., Probstfield, J., Dagenais, G.R., 2005. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama 293, 1338-1347.
Maddock, S.D., Cirulis, M.M., Callahan, S.J., Keenan, L.M., Pirozzi, C.S., Raman, S.M., Aberegg, S.K., 2019. Pulmonary Lipid-Laden Macrophages and Vaping. N Engl J Med.
Matsumoto, S., Fang, X., Traber, M.G., Jones, K.D., Langelier, C., Hayakawa Serpa, P., Calfee, C.S., Matthay, M.A., Gotts, J.E., 2020. Dose-Dependent Pulmonary Toxicity of Aerosolized Vitamin E Acetate. Am J Respir Cell Mol Biol 63 (6), 748-757.
Matsumoto, S., Traber, M.G., Leonard, S.W., Choi, J., Fang, X., Maishan, M., Wick, K.D., Jones, K.D., Calfee, C.S., Gotts, J.E., Matthay, M.A., 2022. Aerosolized vitamin E acetate causes oxidative injury in mice and in alveolar macrophages. Am J Physiol Lung Cell Mol Physiol 322 (6), L771-L783.
McCary, C.A., Yoon, Y., Panagabko, C., Cho, W., Atkinson, J., Cook-Mills, J.M., 2012. Vitamin E isoforms directly bind PKCalpha and differentially regulate activation of PKCalpha. Biochem J 441 (1), 189-198.
Melhorn, D.K., Gross, S., Lake, G.A., Leu, J.A., 1971. The hydrogen peroxide fragility test and serum toco- pherol level in anemias of various etiologies. Blood 37, 438-446.
Mene-Saffrane, L., 2017. Vitamin E Biosynthesis and Its Regulation in Plants. Antioxidants (Basel) 7 (1).
Meydani, M., Koga, T., Ali, S., 2001. Vitamin E deficiency, Encyclopedia of Life Sciences. Nature Publishing Group, pp. 1-6.
Milman, U., Blum, S., Shapira, C., Aronson, D., Miller-Lotan, R., Anbinder, Y., Alshiek, J., Bennett, L., Kostenko, M., Landau, M., Keidar, S., Levy, Y., Khemlin, A., Radan, A., Levy, A.P., 2008. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arterioscler Thromb Vasc Biol 28 (2), 341-347.
Mocchegiani, E., Costarelli, L., Giacconi, R., Malavolta, M., Basso, A., Piacenza, F., Ostan, R., Cevenini, E., Gonos, E.S., Franceschi, C., Monti, D., 2014. Vitamin E-gene interactions in aging and inflammatory age-related diseases: implications for treatment. A systematic review. Ageing Res Rev 14, 81-101.
Mohd Zaffarin, A.S., Ng, S.F., Ng, M.H., Hassan, H., Alias, E., 2020. Pharmacology and Pharmacokinetics of Vitamin E: Nanoformulations to Enhance Bioavailability. Int J Nanomedicine 15, 9961-9974.
Montoya-Arroyo, A., Frank, J., 2024. Revising the nomenclature for vitamin E requires agreement on its vitamin function. Free Radic Biol Med 215, 77-78.
Morrissey, P.A., Sheehy, P.J.A., 1999. Optimal nutrition: vitamin E. Proceedings of the Nutrition Society 58, 459-468.
Morrow, J.D., Frei, B., Longmire, A.W., Gaziano, J.M., Lynch, S.M., Shyr, Y., Strauss, W.E., Oates, J.A., Roberts, L.J., 2nd, 1995. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med 332 (18), 1198-1203.
Muller-Schmehl, K., Beninde, J., Finckh, B., Florian, S., Dudenhausen, J.W., Brigelius-Flohe, R., Schuelke, M., 2004. Localization of alpha-tocopherol transfer protein in trophoblast, fetal capillaries' endothelium and amnion epithelium of human term placenta. Free Radic Res 38 (4), 413-420.
Muller, P.Y., Netscher, T., Frank, J., Stoecklin, E., Rimbach, G., Barella, L., 2005. Comparative quantification of pharmacodynamic parameters of chiral compounds (RRR- vs. all-rac-alpha tocopherol) by global gene expression profiling. J Plant Physiol 162, 811-817.
Munteanu, A., Taddei, M., Tamburini, I., Bergamini, E., Azzi, A., Zingg, J.M., 2006. Antagonistic Effects of Oxidized Low Density Lipoprotein and {alpha}-tocopherol on CD36 Scavenger Receptor Expression in Monocytes: Involvement of protein kinase B and peroxisome proliferator-activated receptor-{gamma}. J Biol Chem 281, 6489-6497.
Munteanu, A., Zingg, J.M., 2007. Cellular, molecular and clinical aspects of vitamin E on atherosclerosis prevention. Molecular Aspects of Medicine 28, 538-590.
Mykhalkiv, M., Denefil, O., Boyarchuk, O., 2024. Vitamin E discussion forum. Can only alpha-tocopherol be called vitamin E, or also other tocochromanols? Free Radic Biol Med 214, 171-172.
Nagashimada, M., Ota, T., 2019. Role of vitamin E in nonalcoholic fatty liver disease. IUBMB Life 71 (4), 516-522.
Nakatomi, T., Itaya-Takahashi, M., Horikoshi, Y., Shimizu, N., Parida, I.S., Jutanom, M., Eitsuka, T., Tanaka, Y., Zingg, J.M., Matsura, T., Nakagawa, K., 2023. The difference in the cellular uptake of tocopherol and tocotrienol is influenced by their affinities to albumin. Sci Rep 13 (1), 7392.
Neuhouser, M.L., Rock, C.L., Eldrige, A.L., Kristal, A.R., Patterson, R.E., Cooper, D.A., Neumark-Sztainer, D., Cheskin, L.J., Thornquist, M.D., 2001. Serum concen- trations of retinol, α-tocopherol and the carotenoids are influenced by diet, race and obesity in a sam- ple of healthy adolescents. Journal of Nutrition 131, 2184-2191.
Neuzil, J., 2002. Alpha-tocopheryl succinate epitomizes a compound with a shift in biological activity due to pro-vitamin-to-vitamin conversion. Biochem Biophys Res Commun 293, 1309-1313.
Ni, J., Pang, S.T., Yeh, S., 2007. Differential retention of alpha-vitamin E is correlated with its transporter gene expression and growth inhibition efficacy in prostate cancer cells. Prostate 67, 463-471.
Ni, J., Wen, X., Yao, J., Chang, H.C., Yin, Y., Zhang, M., Xie, S., Chen, M., Simons, B., Chang, P., di Sant'agnese, A., Messing, E.M., Yeh, S., 2005. tocopherol-associated protein suppresses prostate cancer cell growth by inhibition of the phosphoinositide 3-kinase pathway. Cancer Res 65, 9807-9816.
Nier, B., Weinberg, P.D., Rimbach, G., Stocklin, E., Barella, L., 2006. Differential gene expression in skeletal muscle of rats with vitamin E deficiency. IUBMB Life 58, 540-548.
Niki, E., Traber, M.G., 2012. A history of vitamin E. Ann Nutr Metab 61 (3), 207-212.
Njoroge, R.N., Unno, K., Zhao, J.C., Naseem, A.F., Anker, J.F., McGee, W.A., Nonn, L., Abdulkadir, S.A., 2017. Organoids model distinct Vitamin E effects at different stages of prostate cancer evolution. Sci Rep 7 (1), 16285.
Noguchi, N., Niki, E., 2024. Vitamin E nomenclature. Is RRR-alpha-tocopherol the only vitamin E? Free Radic Biol Med 221, 257-260.
Oski, F.A., Barness, L.A., 1967. Vitamin E deficiency: a previously unrecognized cause of hemolytic anemia in the premature infant. J Pediatr 70 (2), 211-220.
Paddon-Jones, G., 2022. Emerging Applications of Vitamin E TPGS in Drug Delivery. Pharmaceutical Technology, 20-28.
Panagabko, C., Morley, S., Hernandez, M., Cassolato, P., Gordon, H., Parsons, R., Manor, D., Atkinson, J., 2003. Ligand specificity in the CRAL-TRIO protein family. Biochemistry 42, 6467-6474.
Parker, R.S., Sontag, T.J., Swanson, J.E., 2000. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 277, 531-534.
Passarelli, S., Free, C.M., Shepon, A., Beal, T., Batis, C., Golden, C.D., 2024. Global estimation of dietary micronutrient inadequacies: a modelling analysis. Lancet Glob Health 12 (10), e1590-e1599.
Pearce, B.C., Parker, R.A., Deason, M.E., Qureshi, A.A., Wright, J.J., 1992. Hypocholesterolemic activity of synthetic and natural tocotrienols. J Med Chem 35, 3595-3606.
Pei, R., Mah, E., Leonard, S.W., Traber, M.G., Bruno, R.S., 2015. alpha-tocopherol supplementation reduces 5-nitro-gamma-tocopherol accumulation by decreasing gamma-tocopherol in young adult smokers. Free Radic Res 49 (9), 1114-1121.
Pellicano, M., Bulati, M., Buffa, S., Barbagallo, M., Di Prima, A., Misiano, G., Picone, P., Di Carlo, M., Nuzzo, D., Candore, G., Vasto, S., Lio, D., Caruso, C., Colonna-Romano, G., 2010. Systemic immune responses in Alzheimer's disease: in vitro mononuclear cell activation and cytokine production. J Alzheimers Dis 21 (1), 181-192.
Pfluger, P., Kluth, D., Landes, N., Bumke-Vogt, C., Brigelius-Flohe, R., 2004. Vitamin E: underestimated as an antioxidant. Redox Rep 9, 249-254.
Pignitter, M., Stolze, K., Gartner, S., Dumhart, B., Stoll, C., Steiger, G., Kraemer, K., Somoza, V., 2014. Cold fluorescent light as major inducer of lipid oxidation in soybean oil stored at household conditions for eight weeks. J Agric Food Chem 62 (10), 2297-2305.
Poukka, R.K., Bieri, J.G., 1970. Blood alpha tocopherol: erythrocyte and plasma relationships in vitro and in vivo. Lipids 5 (9), 757-761.
Pratico, D., Tangirala, R.K., Rader, D.J., Rokach, J., FitzGerald, G.A., 1998. Vitamin E suppresses isoprostane generation in vivoand reduces atherosclerosis in ApoE-deficient mice. Nat. Med. 4, 1189-1192.
Pryor, W.A., Stone, K., 1993. Oxidants in cigarette smoke, radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Annals of the New York Academy of Sciences 686, 12-27.
Qian, J., Morley, S., Wilson, K., Nava, P., Atkinson, J., Manor, D., 2005. Intracellular trafficking of vitamin E in hepatocytes: the role of tocopherol transfer protein. J Lipid Res 46, 2072-2082.
Quiroz, A., Belledonne, G., Saavedra, F., Gonzalez, J., Busso, D., 2024. Vitamin E supplementation prevents obesogenic diet-induced developmental abnormalities in SR-B1 deficient embryos. Front Cell Dev Biol 12, 1460697.
Rader, D.J., Brewer, H.B., 1993. Abetalipoproteinemia. New insights into lipoprotein assemby and vitamin E metabolism from a rare genetic disease. Journal of the American Medical Association 270, 865-869.
Raederstorff, D., Wyss, A., Calder, P.C., Weber, P., Eggersdorfer, M., 2015. Vitamin E function and requirements in relation to PUFA. Br J Nutr 114 (8), 1113-1122.
Ranard, K.M., Erdman, J.W., Jr., 2018. Effects of dietary RRR alpha-tocopherol vs all-racemic alpha-tocopherol on health outcomes. Nutr Rev 76 (3), 141-153.
Ranard, K.M., Kuchan, M.J., Bruno, R.S., Juraska, J.M., Erdman, J.W., 2020. Synthetic alpha-tocopherol, Compared with Natural alpha-tocopherol, Downregulates Myelin Genes in Cerebella of Adolescent Ttpa-null Mice. J Nutr 150 (5), 1031-1040.
Ranard, K.M., Kuchan, M.J., Erdman, J.W., Jr., 2019. alpha-tocopherol, but Not gamma-tocopherol, Attenuates the Expression of Selective Tumor Necrosis Factor-Alpha-Induced Genes in Primary Human Aortic Cell Lines. Lipids 54 (5), 289-299.
Ranard, K.M., Kuchan, M.J., Juraska, J.M., Erdman, J.W., Jr., 2021. Natural and Synthetic alpha-tocopherol Modulate the Neuroinflammatory Response in the Spinal Cord of Adult Ttpa-null Mice. Curr Dev Nutr 5 (3), nzab008.
Reboul, E., 2018. Vitamin E intestinal absorption: Regulation of membrane transport across the enterocyte. IUBMB Life.
Refat, M., Moore, T.J., Kazui, M., Risby, T.H., Perman, J.A., Schwarz, K.B., 1991. Utility of breath ethane as a noninvasive biomarker of vitamin E status in chil- dren. Pediatric Research 30, 396-403.
Regner-Nelke, L., Nelke, C., Schroeter, C.B., Dziewas, R., Warnecke, T., Ruck, T., Meuth, S.G., 2021. Enjoy Carefully: The Multifaceted Role of Vitamin E in Neuro-Nutrition. Int J Mol Sci 22 (18).
Reilly, M., Delanty, N., Lawson, J.A., Fitzgerald, G., 1996. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation 94, 19-25.
Rhodes, J.S., Rendeiro, C., Mun, J.G., Du, K., Thaman, P., Snyder, A., Pinardo, H., Drnevich, J., Chandrasekaran, S., Lai, C.S., Schimpf, K.J., Kuchan, M.J., 2020. Brain alpha-tocopherol Concentration and Stereoisomer Profile Alter Hippocampal Gene Expression in Weanling Mice. J Nutr 150 (12), 3075-3085.
Ricciarelli, R., Argellati, F., Pronzato, M.A., Domenicotti, C., 2007. Vitamin E and neurodegenerative disease. Molecular Aspects of Medicine 28 (5-6), 591-606.
Ricciarelli, R., Zingg, J.M., Azzi, A., 2000. Vitamin E reduces the uptake of oxidized LDL by inhibiting CD36 scavenger receptor expression in cultured aortic smooth muscle cells. Circulation 102, 82-87.
Rimbach, G., Moehring, J., Huebbe, P., Lodge, J.K., 2010. Gene-regulatory activity of alpha-tocopherol. Molecules 15 (3), 1746-1761.
Roberts, L.J., 2nd, Oates, J.A., Linton, M.F., Fazio, S., Meador, B.P., Gross, M.D., Shyr, Y., Morrow, J.D., 2007. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med 43 (10), 1388-1393.
Robinson, I., de Serna, D.G., Gutierrez, A., Schade, D.S., 2006. Vitamin E in humans: an explanation of clinical trial failure. Endocr Pract 12, 576-582.
Rota, C., Rimbach, G., Minihane, A.M., Stoecklin, E., Barella, L., 2005. Dietary vitamin E modulates differential gene expression in the rat hippocampus: potential implications for its neuroprotective properties. Nutr Neurosci 8, 21-29.
Saleh, M.M., Woods, A., Harvey, R.D., Young, A.R., Jones, S.A., 2021. Nanomaterials fusing with the skin: Alpha-tocopherol phosphate delivery into the viable epidermis to protect against ultraviolet radiation damage. Int J Pharm 594, 120000.
Santander, N., Lizama, C., Murgas, L., Contreras, S., Martin, A.J.M., Molina, P., Quiroz, A., Rivera, K., Salas-Perez, F., Godoy, A., Rigotti, A., Busso, D., 2018. Transcriptional profiling of embryos lacking the lipoprotein receptor SR-B1 reveals a regulatory circuit governing a neurodevelopmental or metabolic decision during neural tube closure. BMC Genomics 19 (1), 731.
Santander, N., Lizama, C., Parga, M.J., Quiroz, A., Perez, D., Echeverria, G., Ulloa, L., Palma, V., Rigotti, A., Busso, D., 2017. Deficient Vitamin E Uptake During Development Impairs Neural Tube Closure in Mice Lacking Lipoprotein Receptor SR-BI. Sci Rep 7 (1), 5182.
Santander, N.G., Contreras-Duarte, S., Awad, M.F., Lizama, C., Passalacqua, I., Rigotti, A., Busso, D., 2013. Developmental abnormalities in mouse embryos lacking the HDL receptor SR-BI. Hum Mol Genet 22 (6), 1086-1096.
Sauberlich, H., 1999. Laboratory Tests for the Assess- ment of Nutritional Status. CRC Press, Cleveland, OH.
Schaefer L, O.K., 1990. Subcutaneous adipose- tissue fatty acids and vitamin E in humans: relation to diet and sampling site. American Journal of Clinical Nutrition 52, 486-490.
Schmolz, L., Birringer, M., Lorkowski, S., Wallert, M., 2016. Complexity of vitamin E metabolism. World J Biol Chem 7 (1), 14-43.
Schuelke, M., Elsner, A., Finckh, B., Kohlschutter, A., Hubner, C., Brigelius-Flohe, R., 2000. Urinary alpha-tocopherol metabolites in alpha-tocopherol transfer protein-deficient patients. J Lipid Res 41 (10), 1543-1551.
Schultz, M., Leist, M., Petrzika, M., Gassmann, B., Brigelius-Flohe, R., 1995. Novel urinary metabolite of alpha-tocopherol, 2,5,7,8-tetramethyl-2(2'-carboxyethyl)-6-hydroxychroman, as an indicator of an adequate vitamin E supply? Am. J. Clin. Nutr. 62, 1527S-1534S.
Shahidi, F., de Camargo, A.C., 2016. tocopherols and tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int J Mol Sci 17 (10).
Sharma, G., Muller, D.P., O'Riordan, S.M., Bryan, S., Dattani, M.T., Hindmarsh, P.C., Mills, K., 2013. Urinary conjugated alpha-tocopheronolactone--a biomarker of oxidative stress in children with type 1 diabetes. Free Radic Biol Med 55, 54-62.
Siger, A., Gornas, P., 2023. Free tocopherols and tocotrienols in 82 plant species' oil: Chemotaxonomic relation as demonstrated by PCA and HCA. Food Res Int 164, 112386.
Simon, E.J., 1956. The metabolism of vitamin E. II. Purification and characterization of urinary metabolites of alpha-tocopherol. J. Biol. Chem. 221, 807-817.
Simon, E.J., Gross, C.S., Milhorat, A.T., 1956. The metabolism of vitamin E. The absorption and excretion of d-a-tocopheryl-5-methyl-C14-succinate. J. Biol. Chem. 221, 797-805.
Smith, M.T., Wyse, B.D., Edwards, S.R., El-Tamimy, M., Gaetano, G., Gavin, P., 2015. Topical application of a novel oxycodone gel formulation (tocopheryl phosphate mixture) in a rat model of peripheral inflammatory pain produces localized pain relief without significant systemic exposure. J Pharm Sci 104 (7), 2388-2396.
Sokol, R.J., 1993. Vitamin E deficiency and neurologi- cal disorders, in: Pacher, L., Fuchs, J. (Eds.), Vitamin E in Health and Disease. Marcel Dekker, New York, pp. 815-849.
Sokol, R.J., 1996. Vitamin E, in: Ziegler, E.E., Filer, L.J. (Eds.), Present Knowledge in Nutrition, 7th ed. International Life Sciences Institute, Washington, DC, pp. 130-136.
Sokol, R.J., Heubi, J.E., Iannaccone, S.T., Bove, K.E., Balistreri, W.F., 1984. Vitamin E deficiency with normal serum vitamin E concentrations in children with chronic cholestasis. N Engl J Med 310, 1209-1212.
Sokol, R.J., Heubi, J.E., Iannaccone, S.T., Bove, K.E., Wf, B., 1983. Mechanism causing vitamin E deficiency during chronic childhood cholestasis. Gastroenterology 85, 1172-1182.
Sokol, R.J., Kim, Y.S., Hoofnagle, J.H., Heubi, J.E., Jones, E.A., Balistreri, W.F., 1989. Intestinal malabsorption of vitamin E in primary biliary cirrhosis. Gastroenterology 96, 479-486.
Somer, S., Levy, A.P., 2020. The Role of Haptoglobin Polymorphism in Cardiovascular Disease in the Setting of Diabetes. Int J Mol Sci 22 (1).
Sontag, T.J., Parker, R.S., 2002. Cytochrome P450 omega-hydroxylase pathway of tocopherol catabolism. Novel mechanism of regulation of vitamin E status. J. Biol. Chem. 277, 25290-25296.
Su, L.C., Bui, M., Kardinaal, A., Gomez-Aracena, J., Martin- Moreno, J., Martin, B., Thamm, M., Simonsen, N., Veer, P., Kok, F., Strain, S., Kohlmeier, L., 1998. Differ- ences between plasma and adipose tissue biomark- ers of carotenoids and tocopherols. Cancer Epidemi- ology. Biomarkers and Prevention 7, 1043-1048.
Sun, Y., Scavini, M., Orlando, R.A., Murata, G.H., Servilla, K.S., Tzamaloukas, A.H., Schrader, R., Bedrick, E.J., Burge, M.R., Abumrad, N.A., Zager, P.G., 2010. Increased CD36 expression signals monocyte activation among patients with type 2 diabetes. Diabetes Care 33 (9), 2065-2067.
Suttorp, N., Toepfer, W., Roka, L., 1986. Antioxidant defense mechanisms of endothelial cells: glutathione redox cycle versus catalase. Am J Physiol 251, C671-680.
Swamynathan, M.M., Kuang, S., Watrud, K.E., Doherty, M.R., Gineste, C., Mathew, G., Gong, G.Q., Cox, H., Cheng, E., Reiss, D., Kendall, J., Ghosh, D., Reczek, C.R., Zhao, X., Herzka, T., Spokaite, S., Dessus, A.N., Kim, S.T., Klingbeil, O., Liu, J., Nowak, D.G., Alsudani, H., Wee, T.L., Park, Y., Minicozzi, F., Rivera, K., Almeida, A.S., Chang, K., Chakrabarty, R.P., Wilkinson, J.E., Gimotty, P.A., Diermeier, S.D., Egeblad, M., Vakoc, C.R., Locasale, J.W., Chandel, N.S., Janowitz, T., Hicks, J.B., Wigler, M., Pappin, D.J., Williams, R.L., Cifani, P., Tuveson, D.A., Laporte, J., Trotman, L.C., 2024. Dietary pro-oxidant therapy by a vitamin K precursor targets PI 3-kinase VPS34 function. Science 386 (6720), eadk9167.
Sylvester, P.W., Akl, M.R., Malaviya, A., Parajuli, P., Ananthula, S., Tiwari, R.V., Ayoub, N.M., 2014. Potential role of tocotrienols in the treatment and prevention of breast cancer. Biofactors 40 (1), 49-58.
Takasaki, A., Tamura, H., Miwa, I., Taketani, T., Shimamura, K., Sugino, N., 2010. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril 93 (6), 1851-1858.
Tamura, T., Otulakowski, G., Kavanagh, B.P., 2019. Could nanotechnology make vitamin E therapeutically effective? Am J Physiol Lung Cell Mol Physiol 316 (1), L1-L5.
Terasawa, Y., Ladha, Z., Leonard, S.W., Morrow, J.D., Newland, D., Sanan, D., Packer, L., Traber, M.G., Farese, R.V., Jr., 2000. Increased atherosclerosis in hyperlipidemic mice deficient in alpha - tocopherol transfer protein and vitamin E. Proc. Natl. Acad. Sci. U.S.A. 97, 13830-13834.
Thiele, J.J., Ekanayake-Mudiyanselage, S., 2007. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol Aspects Med 28 (5-6), 646-667.
Thiele, J.J., Weber, S.U., Packer, L., 1999. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J Invest Dermatol 113, 1006-1010.
Thurnham, D.I., Davies, J.A., Crump, B.J., Situnayake, R.D., Davis, M., 1986. The use of different lipids to express serum tocopherol: lipid ratios for the meas- urement of vitamin E status. Annals of Clinical Biochemistry 23, 514-520.
Tian, H., Li, Y.F., Jiao, G.L., Sun, W.Y., He, R.R., 2024. Unveiling the antioxidant superiority of alpha-tocopherol: Implications for vitamin E nomenclature and classification. Free Radic Biol Med 216, 46-49.
Torquato, P., Giusepponi, D., Bartolini, D., Barola, C., Marinelli, R., Sebastiani, B., Galarini, R., Galli, F., 2021. Pre-analytical monitoring and protection of oxidizable lipids in human plasma (vitamin E and omega-3 and omega-6 fatty acids): An update for redox-lipidomics methods. Free Radic Biol Med 176, 142-148.
Torquato, P., Ripa, O., Giusepponi, D., Galarini, R., Bartolini, D., Wallert, M., Pellegrino, R., Cruciani, G., Lorkowski, S., Birringer, M., Mazzini, F., Galli, F., 2016. Analytical strategies to assess the functional metabolome of vitamin E. J Pharm Biomed Anal 124, 399-412.
Traber, M.G., 2004. Vitamin E, nuclear receptors and xenobiotic metabolism. Arch Biochem Biophys 423, 6-11.
Traber, M.G., 2014. Vitamin E inadequacy in humans: causes and consequences. Adv Nutr 5 (5), 503-514.
Traber, M.G., 2020. Brain-E, Does It Equate to Brainy? J Nutr 150 (12), 3049-3050.
Traber, M.G., 2024a. Deciphering the enigma of the function of alpha-tocopherol as a vitamin. Free Radic Biol Med 221, 64-74.
Traber, M.G., 2024b. Human Vitamin E deficiency, and what is and is not Vitamin E? Free Radic Biol Med 213, 285-292.
Traber, M.G., Burton, G.W., Ingold, K.U., Kayden, H.J., 1990a. RRR- and SRR-alpha-tocopherols are secreted without discrimination in human chylomicrons, but RRR-alpha-tocopherol is preferentially secreted in very low density lipoproteins. J. Lipid. Res. 31, 675-685.
Traber, M.G., Cross, C.E., 2023. Alpha-tocopherol from People to Plants Is an Essential Cog in the Metabolic Machinery. Antioxid Redox Signal 38 (10-12), 775-791.
Traber, M.G., Elsner, A., Brigelius-Flohe, R., 1998. Synthetic as compared with natural vitamin E is preferentially excreted as alpha-CEHC in human urine: studies using deuterated alpha-tocopheryl acetates. FEBS Lett 437 (1-2), 145-148.
Traber, M.G., Head, B., 2021. Vitamin E: How much is enough, too much and why! Free Radic Biol Med 177, 212-225.
Traber, M.G., Kayden, H.J., 1989. Preferential incorporation of alpha-tocopherol vs gamma-tocopherol in human lipoproteins. Am. J. Clin. Nutr. 49, 517-526.
Traber, M.G., Sokol, R.J., Burton, G.W., Ingold, K.U., Papas, A.M., Huffaker, J.E., Kayden, H.J., 1990b. Impaired ability of patients with familial isolated vitamin E deficiency to incorporate alpha-tocopherol into lipoproteins secreted by the liver. J Clin Invest 85, 397-407.
Valk, E.E., Hornstra, G., 2000. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: a review. Int J Vitam Nutr Res 70 (2), 31-42.
van Kempen, T., Benitez Punal, S., Huijser, J., De Smet, S., 2022. tocopherol more bioavailable than tocopheryl-acetate as a source of vitamin E for broilers. PLoS One 17 (5), e0268894.
Vandewoude, M., Claeys, M., Leeuw, I., 1984. Deter- mination of alpha-tocopherol in human plasma by high performance liquid chromatography with elec- trochemical detection. Journal of Chromatography 311, 176-182.
Vandewoude, M.F., Vandewoude, M.G., 1987. Vitamin E status in a normal population: the influence of age. J Am Coll Nutr 6 (4), 307-311.
Vatassery, G.T., Johnson, G.J., Krezowski, A.M., 1983a. Changes in vitamin E concentrations in human plas- ma and platelets with age. Journal of the American College of Nutrition 2, 369-375.
Vatassery, G.T., Krezowski, A.M., Eckfeldt, J.H., 1983b. Vitamin E concentrations in human blood plasma and platelets. Am J Clin Nutr 37 (6), 1020-1024.
Velthuis-te Wierik, E.J., van den Berg, H., Weststrate, J.A., van het Hof, K.H., de Graaf, C., 1996. Consumption of reduced-fat products: effects on parameters of anti-oxidative capacity. Eur J Clin Nutr 50 (4), 214-219.
Villacorta, L., Azzi, A., Zingg, J.M., 2007. Regulatory role of vitamin E and C on extracellular matrix components of the vascular system. Molecular Aspects of Medicine 28, 507-537.
Villacorta, L., Minarrieta, L., Salvatore, S.R., Khoo, N.K., Rom, O., Gao, Z., Berman, R.C., Jobbagy, S., Li, L., Woodcock, S.R., Chen, Y.E., Freeman, B.A., Ferreira, A.M., Schopfer, F.J., Vitturi, D.A., 2018. In situ generation, metabolism and immunomodulatory signaling actions of nitro-conjugated linoleic acid in a murine model of inflammation. Redox Biol 15, 522-531.
Vivarelli, F., Canistro, D., Cirillo, S., Papi, A., Spisni, E., Vornoli, A., Croce, C.M.D., Longo, V., Franchi, P., Filippi, S., Lucarini, M., Zanzi, C., Rotondo, F., Lorenzini, A., Marchionni, S., Paolini, M., 2019. Co-carcinogenic effects of vitamin E in prostate. Sci Rep 9 (1), 11636.
Vries, Y.d., Pundir, S., McKenzie, E., Keijer, J., Kussmann, M., 2018. Maternal Circulating Vitamin Status and Colostrum Vitamin Composition in Healthy Lactating Women-A Systematic Approach. Nutrients 10 (6).
Wagner, K.H., Kamal-Eldin, A., Elmadfa, I., 2004. Gamma-tocopherol--an underestimated vitamin? Ann Nutr Metab 48, 169-188.
Wallert, M., Schmolz, L., Galli, F., Birringer, M., Lorkowski, S., 2014. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol 2, 495-503.
Wechter, W.J., Kantoci, D., Murray, E.D., Jr., D'Amico, D.C., Jung, M.E., Wang, W.H., 1996. A new endogenous natriuretic factor: LLU-alpha. Proc. Natl. Acad. Sci. U.S.A. 93, 6002-6007.
Weiser, H., Riss, G., Kormann, A.W., 1996. Biodiscrimination of the eight alpha-tocopherol stereoisomers results in preferential accumulation of the four 2R forms in tissues and plasma of rats. J. Nutr. 126, 2539-2549.
Weiser, H., Vecchi, M., 1982. Stereoisomers of alpha-tocopheryl acetate. II. Biopotencies of all eight stereoisomers, individually or in mixtures, as determined by rat resorption-gestation tests. Int J Vitam Nutr Res 52, 351-370.
Weiss, W.P., Hogan, J.S., Wyatt, D.J., 2009. Relative bioavailability of all-rac and RRR vitamin E based on neutrophil function and total alpha-tocopherol and isomer concentrations in periparturient dairy cows and their calves. J Dairy Sci 92 (2), 720-731.
Wright, M.E., Peters, U., Gunter, M.J., Moore, S.C., Lawson, K.A., Yeager, M., Weinstein, S.J., Snyder, K., Virtamo, J., Albanes, D., 2009. Association of Variants in Two Vitamin E Transport Genes with Circulating Vitamin E Concentrations and Prostate Cancer Risk. Cancer Res 69 (4), 1429-1438.
Wu, J.H., Hodgson, J.M., Ward, N.C., Clarke, M.W., Puddey, I.B., Croft, K.D., 2005. Nitration of gamma-tocopherol prevents its oxidative metabolism by HepG2 cells. Free Radic Biol Med 39, 483-494.
Yamamoto, Y., Fujisawa, A., Hara, A., Dunlap, W.C., 2001. An unusual vitamin E constituent (alpha-tocomonoenol) provides enhanced antioxidant protection in marine organisms adapted to cold-water environments. Proc. Natl. Acad. Sci. U S A 98, 13144-13148.
Yasunaga, T., Kato, H., Ohgaki, K., Inamoto, T., Hikasa, Y., 1982. Effect of vitamin E as an immunopotentiation agent for mice at optimal dosage and its toxicity at high dosage. J Nutr 112 (6), 1075-1084.
Yazgan, B., Sozen, E., Karademir, B., Ustunsoy, S., Ince, U., Zarkovic, N., Ozer, N.K., 2017. CD36 expression in peripheral blood mononuclear cells reflects the onset of atherosclerosis. Biofactors.
Yazgan, B., Ustunsoy, S., Karademir, B., Kartal-Ozer, N., 2014. CD36 as a biomarker of atherosclerosis. Free Radic Biol Med 75 Suppl 1, S10.
Yin, Y., Dong, Y., Cao, Y., Dong, G., 2024. Association of Vitamin E Intake with All-Cause Mortality Among Individuals with Rheumatoid Arthritis: A Cohort Study from the NHANES 1999-2018. J Am Nutr Assoc, 1-7.
Yokota, T., Uchihara, T., Kumagai, J., Shiojiri, T., Pang, J.J., Arita, M., Arai, H., Hayashi, M., Kiyosawa, M., Okeda, R., Mizusawa, H., 2000. Postmortem study of ataxia with retinitis pigmentosa by mutation of the alpha-tocopherol transfer protein gene. J. Neurol. Neurosurg. Psychiatry 68, 521-525.
Yong, S.T., Wong, H.K., Mardhati, M., Tan, S.L., 2014. tocotreinol and tocopherol contents of annatto seed accessions. Journal of Sicnece and Technology in the Tropics 10, 15-25.
Zhang, Z., Tan, S., Feng, S.S., 2012. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 33 (19), 4889-4906.
Zhao, L., Yagiz, Y., Xu, C., Lu, J., Chung, S., Marshall, M.R., 2015. Muscadine grape seed oil as a novel source of tocotrienols to reduce adipogenesis and adipocyte inflammation. Food Funct 6 (7), 2293-2302.
Zhou, C., Tabb, M.M., Sadatrafiei, A., Grun, F., Blumberg, B., 2004. tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab Dispos 32, 1075-1082.
Zingg, J.M., 2007a. Modulation of signal transduction by vitamin E. Molecular Aspects of Medicine 28, 481-506.
Zingg, J.M., 2007b. Vitamin E: an overview of major research directions. Molecular Aspects of Medicine 28, 400-422.
Zingg, J.M., 2012. Vitamin E and Disease Risk: Research Focus Turns on Genetic Polymorphisms and Molecuar Mechanisms. Vitamins & Trace Elements 1 (3).
Zingg, J.M., 2015. Vitamin E: A Role in Signal Transduction. Annu Rev Nutr 35, 135-173.
Zingg, J.M., 2018. Water-Soluble Vitamin E-tocopheryl Phosphate. Adv Food Nutr Res 83, 311-363.
Zingg, J.M., 2019. Vitamin E: Regulatory Role on Signal Transduction. IUBMB Life 71 (4), 456-478.
Zingg, J.M., 2022. Vitamin E physiology and health effects. Elsevier.
Zingg, J.M., 2024. Finding vitamin Ex(double dagger). Free Radic Biol Med 211, 171-173.
Zingg, J.M., Azzi, A., 2004. Non-antioxidant activities of vitamin E. Cur. Med. Chem. 11, 1113-1133.
Zingg, J.M., Azzi, A., 2009. Comment re: Vitamin E transport gene variants and prostate cancer. Cancer Res 69 (16), 6756; author reply 6756.
Zingg, J.M., Azzi, A., Meydani, M., 2008a. Genetic polymorphisms as determinants for disease-preventive effects of vitamin E. Nutr Rev 66 (7), 406-414.
Zingg, J.M., Azzi, A., Meydani, M., 2015. Induction of VEGF expression by alpha-tocopherol and alpha-tocopheryl phosphate via PI3Kgamma/PKB and hTAP1/SEC14L2-mediated lipid exchange. J Cell Biochem 116, 398-407.
Zingg, J.M., Azzi, A., Meydani, M., 2017. alpha-tocopheryl Phosphate Induces VEGF Expression via CD36/PI3Kgamma in THP-1 Monocytes. J Cell Biochem 118 (7), 1855-1867.
Zingg, J.M., Han, S.N., Pang, E., Meydani, M., Meydani, S.N., Azzi, A., 2013. In vivo regulation of gene transcription by alpha- and gamma-tocopherol in murine T lymphocytes. Arch Biochem Biophys 538 (2), 111-119.
Zingg, J.M., Kempna, P., Paris, M., Reiter, E., Villacorta, L., Cipollone, R., Munteanu, A., De Pascale, C., Menini, S., Cueff, A., Arock, M., Azzi, A., Ricciarelli, R., 2008b. Characterization of three human sec14p-like proteins: alpha-tocopherol transport activity and expression pattern in tissues. Biochimie 90 (11-12), 1703-1715.
Zingg, J.M., Meydani, M., 2019. Interaction between Vitamin E and Polyunsaturated Fatty Acids, in: Weber, e.a. (Ed.), Vitamin E in Human Health. Springer Nature Switzerland AG, Switzerland, pp. 141-159.
Zingg, J.M., Vlad, A., Ricciarelli, R., 2021. Oxidized LDLs as Signaling Molecules. Antioxidants (Basel) 10 (8).