Gibson RS1,
Principles of
Nutritional Assessment:
Niacin
3rd Edition August 2024
Abstract
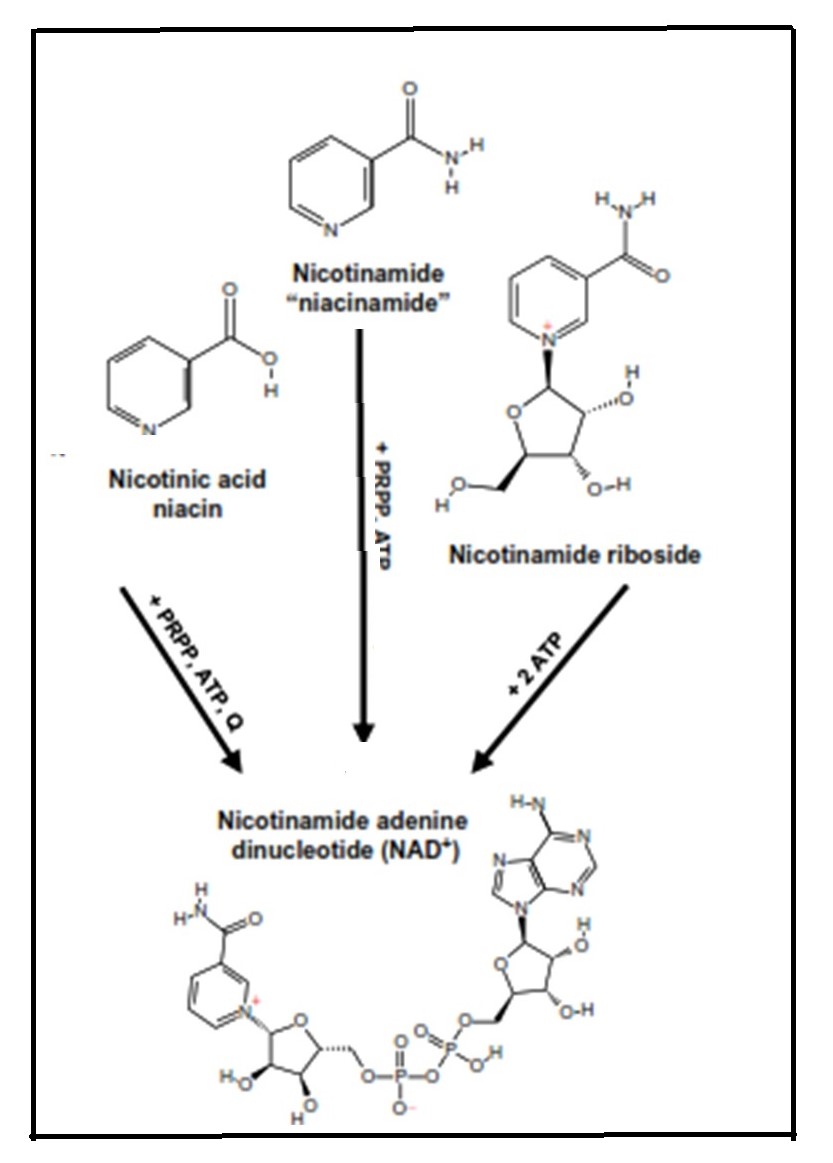
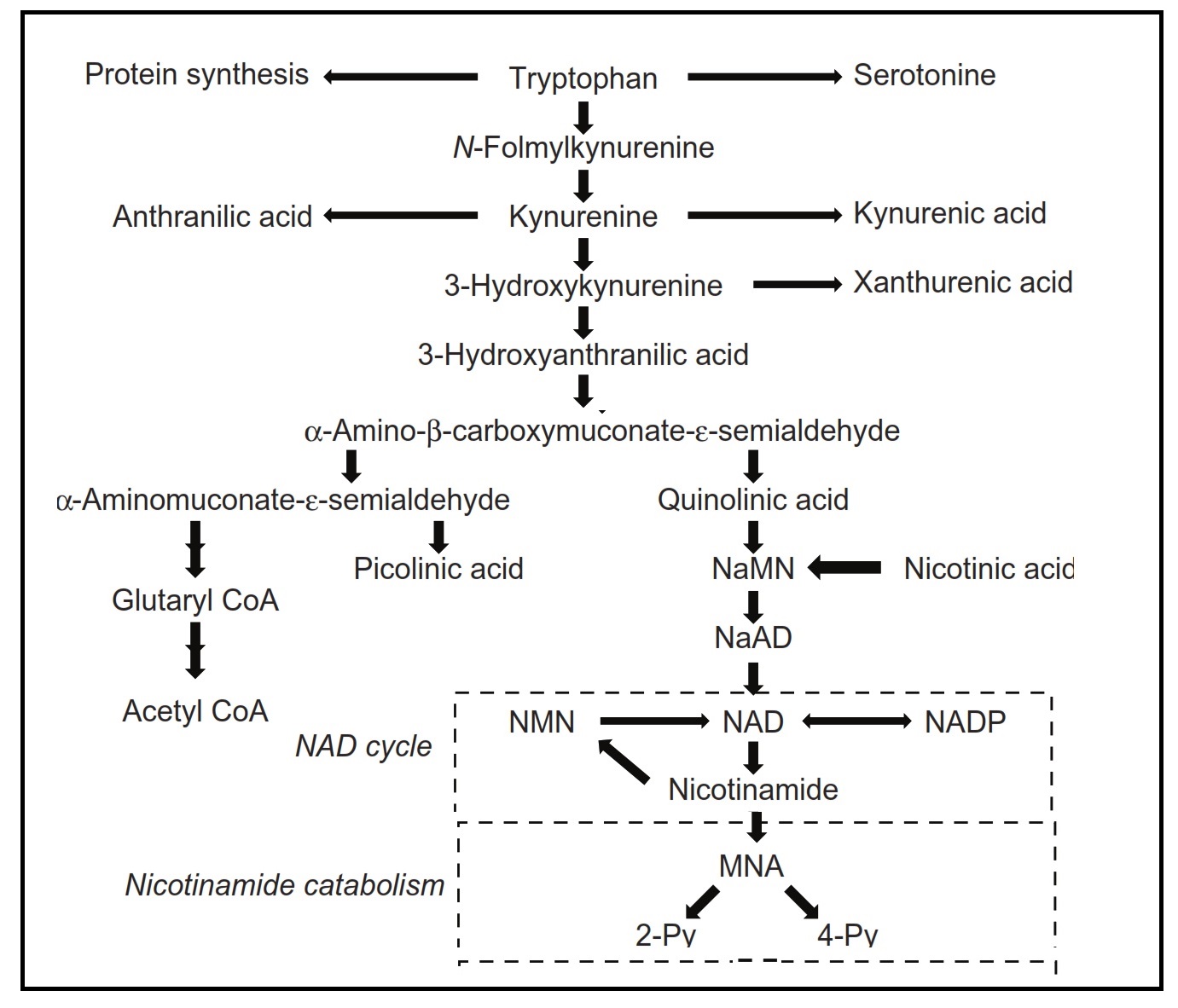
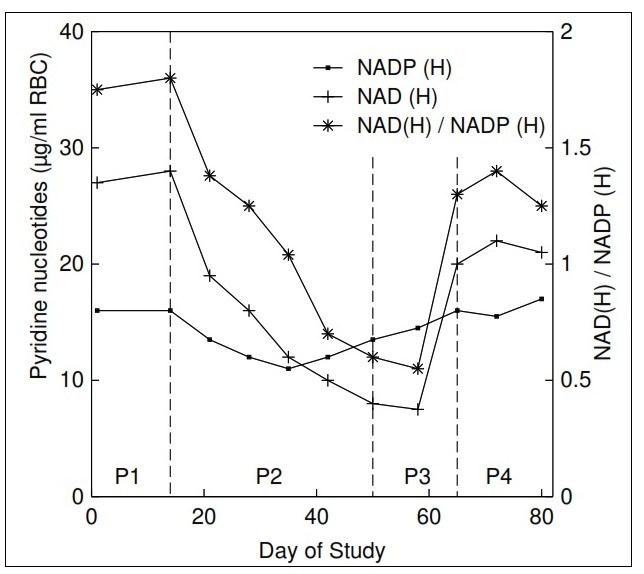
The term "niacin" refers to three compounds: nicotinamide, nicotinic acid, and nicotinamide ribose. They are precursors of nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP), both of which function in a variety of oxidation and reduction reactions and are associated with both catabolic and anabolic processes. Pellagra, a disease of the "4Ds" (diarrhea, dermatitis, dementia, and death) is the syndrome of niacin deficiency. Although rare, outbreaks of pellagra still occur when diets are based on maize (without nixtamalization), a cereal low in bioavailable niacin. Secondary niacin deficiency may also develop in diseases that interfere with absorption of niacin or tryptophan (a precursor of niacin) (e.g., chronic colitis, liver cirrhosis) or reduce the conversion of tryptophan to niacin (e.g., chronic alcoholism, prolonged isoniazid treatment for tuberculosis). Rich food sources of niacin include meat, poultry, and fish, followed by dairy products and enriched or fortified cereals. Coffee is also a good source of niacin. Absorption of niacin from food ranges from about 23% to 70% and is lowest from cereals and highest from animal products. Certain food preparation and processing practices may influence the content and bioavailability of niacin in food. Intakes and requirements for niacin are expressed as niacin equivalents (NEs) to consider the contribution of tryptophan to niacin (i.e., 60mg tryptophan yields 1mg niacin). Dietary reference values for niacin are set as NE per day, or alternatively expressed as mg NE/MJ or mg NE/1000 kcal based on the relationship between the requirements for niacin and energy. Niacin toxicity may occur when large pharmacological doses of niacin are taken to prevent or treat metabolic diseases such as dyslipidemia. Tolerable Upper Intake Levels (ULs) are based on dermal vasodilative flushing. At present, the best biomarker of niacin status is the measurement of two urinary metabolites: N'‑methylnicotinamide and N'‑methyl-2-pyridine-5-carboxamide (2‑pyridone) using HPLC. Sampling strategies employed include 24h urines with results expressed as concentrations of individual metabolites (mg/d), or random spot urines when the ratio (2‑pyridone to N'‑methylnicotinamide), or concentrations relative to creatinine (mg/g creatinine) are used. Interpretive criteria for adults are available for urinary excretion of these niacin metabolites expressed relative to creatinine and as the ratio (2‑pyridone / N'‑methylnicotinamide). WHO has developed provisional criteria for the severity of public health problem of niacin deficiency. Concentrations of the co-enzyme NAD in erythrocytes (but not whole blood) or the ratio of erythrocyte NAD to NADP (called niacin index) are said to be sensitive to short-term changes in niacin intake, even in the absence of clinical signs of deficiency. Hence, they may serve as a sensitive and reliable functional biomarker for risk of niacin deficiency. With the development of a rapid LC-MS/MS method for their analysis, further investigation of erythrocyte NAD and NADP concentrations as biomarkers of niacin status is warranted. CITE AS: Gibson RS. Principles of Nutritional Assessment. Niacin https://nutritionalassessment.org/niacin/Email: Rosalind.Gibson@Otago.AC.NZ
Licensed under CC-BY-4.0
( PDF ).